According to a University of Macau (UM) statement on Friday, a research team from the public university has discovered a drug combination that enhances the efficacy of proteasome inhibitors* against solid tumours and improves immune checkpoint inhibitor therapy for breast cancer.
The study, led by Deng Chuxia, chair professor in the Faculty of Health Sciences (FHS) at UM, used drug combination screening to identify a new strategy for proteasome inhibitor-based therapy.
While proteasome inhibitors such as bortezomib** are widely used to treat liquid tumours like multiple myeloma***, they have shown limited effectiveness against solid tumours in vivo despite high sensitivity in vitro, the statement said, as previous UM research revealed that breast cancer cells resistant to cisplatin**** increase proteasome activity to evade cell death, and combining proteasome inhibitors could reverse multidrug resistance.
To address the limitations in solid tumours, the statement noted that the team screened for agents that could enhance the tumour-killing effect of bortezomib and identified two FDA-approved drugs — ammonium tetrathiomolybdate (TM)***** and plerixafor******. These drugs sensitise breast cancer cells to bortezomib by modulating the AMPK/STAT3/PSMB5 signalling axis.
The statement also said that in mouse models, the combination worked in a CD8+ T cell*******-dependent manner by inducing tumour cells to secrete CCL5******** to recruit CD8+ T cells, activating the antigen processing and presentation pathway, and enabling CD8+ T cells to kill tumour cells and strengthen the anti-tumour immune response.
The research, published in the international journal Cell Reports Medicine, presents a clinically promising strategy for improving proteasome inhibitor-based therapies against solid tumours, as the statement concluded that the study was conducted by Prof. Deng as corresponding author with first author Tang Dongyang and contributions from multiple FHS members, supported by the National Natural Science Foundation of China, the Science and Technology Development Fund of the Macao SAR, and the University of Macau.
*Proteasome Inhibitors are a class of drugs that block the activity of proteasomes, which are large protein complexes responsible for degrading damaged or unwanted proteins in cells. By inhibiting proteasome function, these drugs disrupt protein homeostasis, leading to the accumulation of misfolded or harmful proteins, ultimately causing cell death—particularly in rapidly dividing cells like cancer cells – DeepSeek
**Bortezomib is a proteasome inhibitor used primarily in the treatment of multiple myeloma and mantle cell lymphoma – DeepSeek
***Multiple myeloma (MM) is a blood cancer that affects plasma cells (a type of white blood cell that produces antibodies). In myeloma, these cells grow uncontrollably in the bone marrow, crowding out healthy cells and causing bone damage, kidney problems, and weakened immunity. – DeepSeek
****Cisplatin is a chemotherapy drug used to treat various cancers by damaging cancer cell DNA, preventing them from multiplying. – DeepSeek
*****Ammonium tetrathiomolybdate (TM) is a copper-binding drug used to treat Wilson’s disease (a disorder where too much copper builds up in the body). It works by trapping excess copper and helping remove it. – DeepSeek
******Plerixafor is a drug used to help collect stem cells from the blood for bone marrow transplants, especially in patients with multiple myeloma or lymphoma. – DeepSeek
*******CD8+ T cells (also called cytotoxic T lymphocytes, CTLs) are a subset of T cells that play a critical role in the adaptive immune system by identifying and killing infected or cancerous cells. – DeepSeek
********CCL5 (also known as RANTES, Regulated on Activation, Normal T-cell Expressed and Secreted) is a chemokine that plays a key role in immune cell recruitment, inflammation, and antiviral responses. – DeepSeek
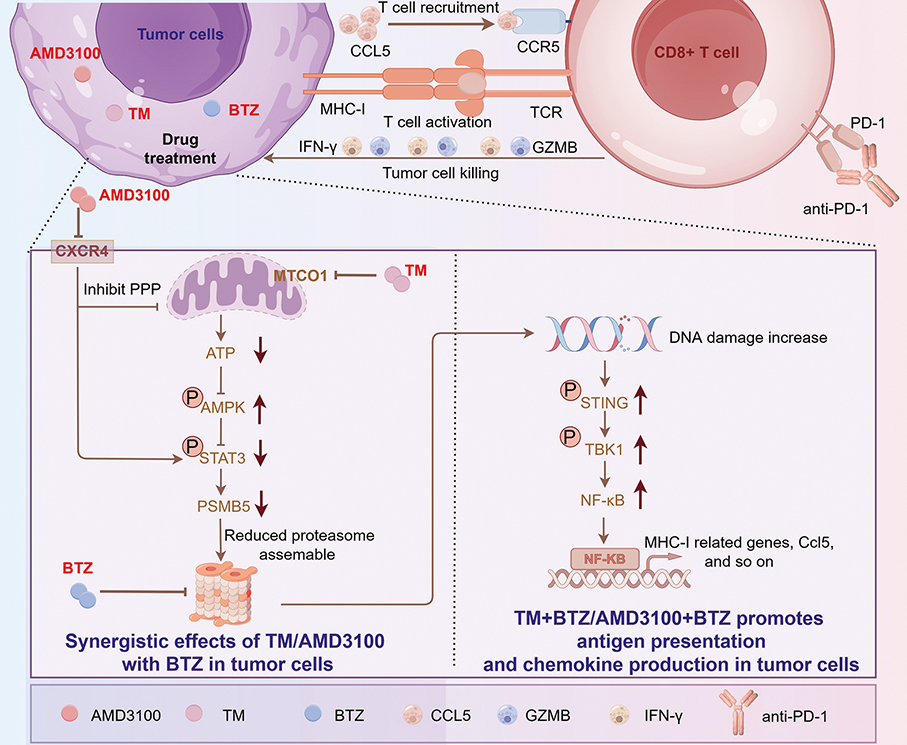
This infographic provided by UM shows the mechanisms by which TM and plerixafor sensitise bortezomib’s therapeutic effect, with the drug combination inducing the activation of anti-tumour immunity.






